Inclusion of
Pregnant and
Lactating People
in Clinical Trials.
Providers caring for pregnant and lactating people make decisions based on limited, and sometimes no evidence.
Pregnant and lactating people have been systematically excluded from many clinical trials, including recent vaccine and therapeutics trials. Up to 70% of pregnant and lactating people take at least one prescription medication, despite limited and sometimes no clinical trials of these medications in pregnancy or lactation. As a result, providers caring for pregnant and lactating people make decisions based on limited and sometimes no evidence.
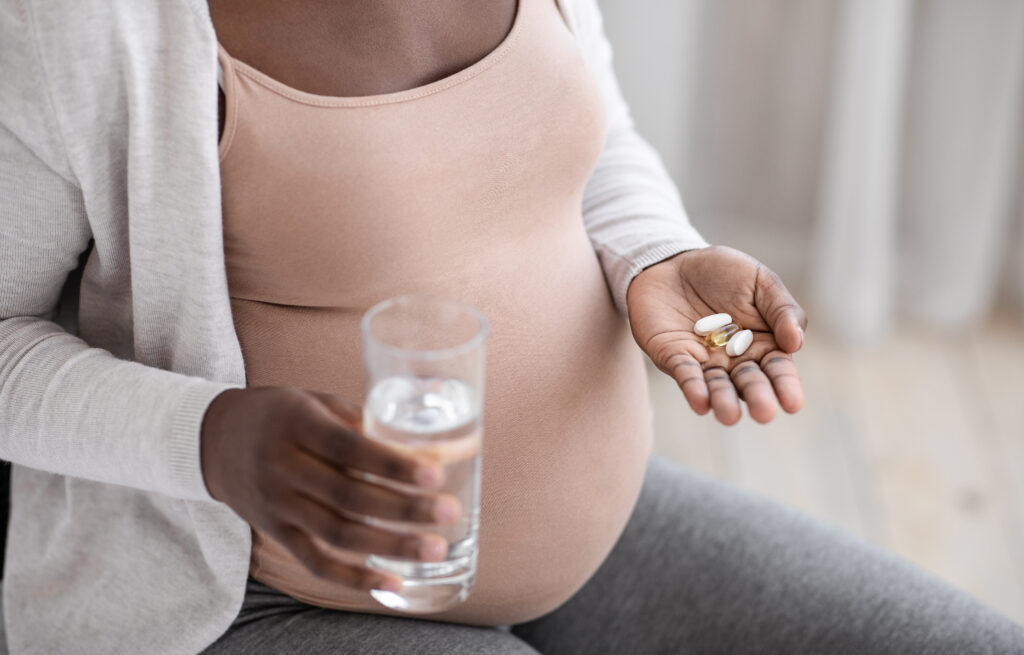

Facilitating safe access to evidence-based treatments and medical care for pregnant women.
PREGTRIAL is a national team of researchers, knowledge users, Non-Governmental Organizations (NGOs), key stakeholders, Indigenous persons representatives, and people with lived experience, working to ensure pregnant and lactating people have access to safe, evidence-based, and culturally sensitive medical care.
We aim to improve the participation and representation of pregnant and lactating people in clinical trials through a multi-faceted strategy that builds on existing pan-Canadian initiatives and training platforms.


Our Aims and Approach
Evidence Review
and Synthesis
We are reviewing evidence from available clinical trials over the last decade on inclusion or exclusion of pregnant and lactating people and of reproductive aged women. This will help us understand current practices and advocate for future change.
Support the development of Canada-specific guidance for the conduct of clinical trials including pregnant and lactating people.
Assess investigator and research ethics board-related barriers, facilitators, and needs to support the responsible inclusion of pregnant and lactating people in clinical trials.
Development of
Tools
We are co-developing tools such as an adapted informed consent form template and other toolkits using a multi-pronged approach to ensure that the ethics surrounding inclusion of pregnant and lactating people are adequately captured in contemporary research.













We Want to Hear From You
Are you a healthcare professional, member of a trial team, research ethics board, industry, or regulator, please take our survey on RedCap.