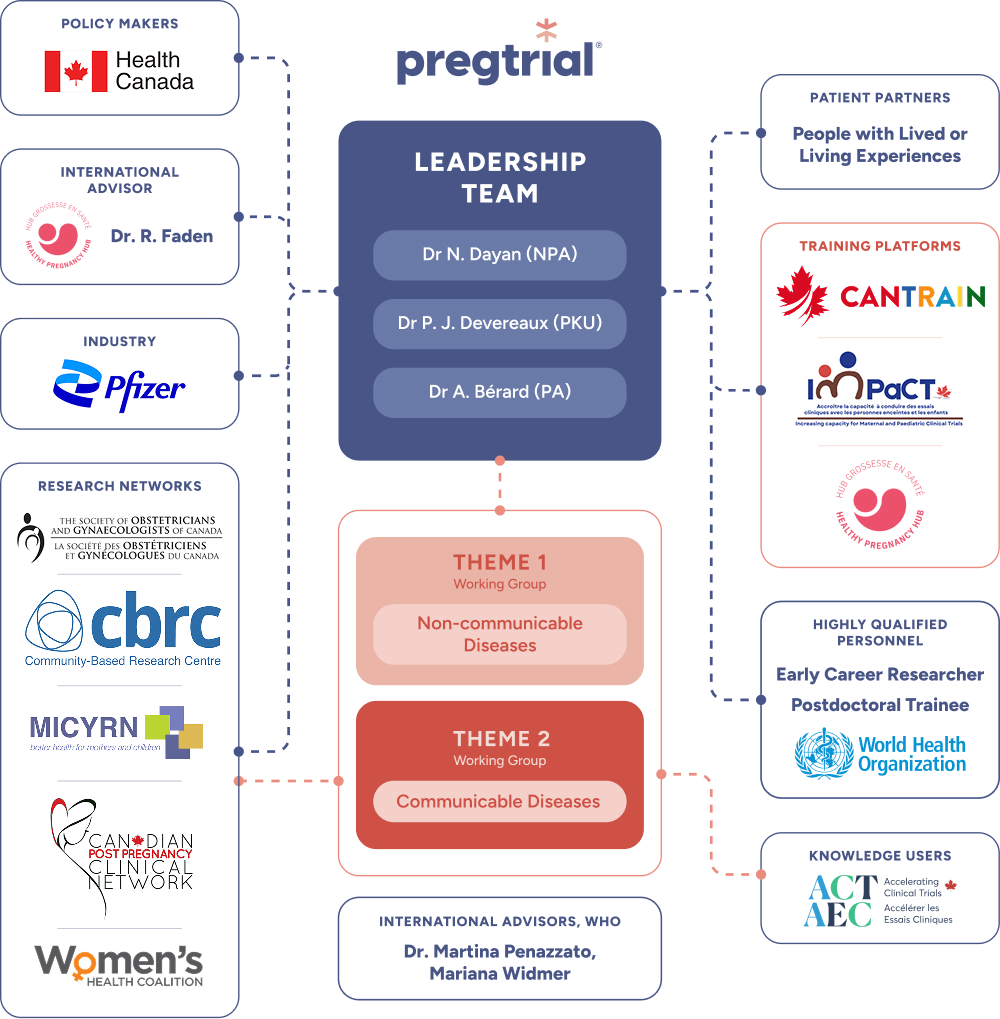
PregTrial Team
The PREGTRIAL team includes clinicians, researchers, public health experts, patient representatives, industry partners, and policymakers, all working together to improve clinical trial inclusion for pregnant and lactating people in Canada.
Leadership Team

Dr. Natalie Dayan is a physician at the McGill University Health Centre (MUHC), where she leads the Obstetric Medicine program. She is also a mid-career scientist at the Research Institute of MUHC (RI-MUHC) specializing in maternal health specifically and women’s health more broadly. Her work is supported by the Fonds de Recherche du Québec – Santé
Dr. Dayan brings extensive experience from her roles as former Early Career Professor in Women’s Heart and Brain Health (Quebec), principal investigator of the multi-centre behavioural Randomized Controlled Trial (RCT) “she MATTERS,” and lead author of the pregnancy section of the Canadian Cardiovascular Society Lipid Guidelines, among other national funded projects in women’s health. She is also actively involved in key networks, including CAMCCO, IMPACT, and the Post-Pregnancy Clinical Network, giving her unique insight into addressing gaps in knowledge and care for pregnant and lactating individuals.

Dr. Devereaux is a cardiologist, perioperative care physician, and clinical epidemiologist. He serves as the Director of the Division of Perioperative Care at McMaster University and is a Senior Scientist as well as the Scientific Leader of the Anesthesiology, Perioperative Medicine, and Surgical Research Group at the Population Health Research Institute (PHRI). He is also the Nominated Principal Applicant of the Accelerating Clinical Trials (ACT) Consortium, the Pan-Canadian Clinical Trials Consortium funded by the Canadian Institutes of Health Research (CIHR). In addition, he holds a Tier 1 Canadian Research Chair in Perioperative Medicine.
A full Professor at McMaster University, Dr. Devereaux is also President of the Society of Perioperative Research and Care. He has published over 480 peer-reviewed papers and more than 93 book chapters, editorials, and commentaries, including 17 in the New England Journal of Medicine, 13 in The Lancet, and 11 in JAMA. He holds an h-index of 127 and has delivered over 1,100 lectures and research presentations across 41 countries. His research program centers on major vascular complications during and after surgery, and he has led numerous large international randomized trials and prospective cohort studies focused on major perioperative vascular complications.

Dr. Anick Bérard has cross-training in epidemiology, pharmacoepidemiology, and genetics from McGill University, Harvard Medical School, and Stanford University. She is a full professor of perinatal epidemiology at the University of Montreal’s Faculty of Pharmacy and CHU Ste-Justine in Montreal, and an adjunct professor at the Faculty of Medicine of Université Claude Bernard in Lyon, France.
She holds a Tier 1 Canada Research Chair in Medications and Pregnancy, as well as the Louis-Boivin Research Chair on Medications, Pregnancy, and Lactation at the University of Montreal. Dr. Bérard also serves as Director of the Research Unit on Medications and Pregnancy at CHU Ste-Justine and Director of the FRQS Réseau Québécois de recherche sur les médicaments. She is a Fellow of both the Canadian Academy of Health Sciences and the International Society of Pharmacoepidemiology, and a voting member of the Birth Defects Research Society.
Dr. Bérard is the principal investigator of several major research projects, including:
She has published over 500 scientific papers, abstracts, and patents, and has secured over $37 million in research funding from CIHR, CFI, and FRQS as principal investigator. Her outstanding contributions have been recognized with the Most Distinguished Scientist Award from the International Teratology Society for her work on antidepressants, maternal depression, and pregnancy, as well as a Research Career Award from the Association of Faculties of Pharmacy of Canada.
Dr. Bérard also serves on the Scientific and Ethics Committee of the Cohorte Marianne in France, a national study investigating epigenetic, environmental, and lifestyle factors contributing to neurodevelopmental disorders in children from in utero to six years of age.
Research Co-Investigators
This team is made up of thematic champions who have expertise in Noncommunicable diseases (NCDs) in pregnancy and lactation, and communicable diseases (CD) in pregnancy. The team includes experts in patient-oriented research, adaptive pediatric trial designs, neonatal surveillance trial bio-ethics and Consolidated Framework for Implementation Research (CFIR).

Dr. Scott Halperin is a Professor of Pediatrics and Microbiology & Immunology at Dalhousie University in Halifax, Nova Scotia. He serves as Director of the Canadian Center for Vaccinology (CCfV) and is the Nominated Principal Investigator of the Canadian Immunization Research Network (CIRN). He previously held the role of Co-Principal Investigator for the Immunization Monitoring Program–Active (IMPACT) and was a member of the Executive Committee of the Canadian Association for Immunization Research and Evaluation (CAIRE).
Dr. Halperin has played a pivotal role in building and leading collaborative vaccine research networks across Canada, generating research that directly informs public health policy and immunization practices. A leading expert in pertussis, his work spans the diagnosis, treatment, and prevention of pertussis and other vaccine-preventable diseases.
His research team has conducted more than 250 clinical trials, including over 20 phase 1 studies. These have covered early-phase vaccine trials for SARS-CoV-2, Ebola virus, group A Streptococcus, Bordetella pertussis, influenza, and respiratory syncytial virus (RSV), as well as later-phase studies for HPV, varicella-zoster virus, meningococcal B, and hepatitis B. Dr. Halperin is a trusted advisor to the vaccine industry and has served on numerous advisory committees for vaccine manufacturers.

Dr. Lauren Kelly is an assistant professor in pharmacology and therapeutics at the University of Manitoba. She is a scientist at the Children’s Hospital Research Institute of Manitoba, a certified clinical research professional and a clinical trialist at the George and Fay Yee Centre for Healthcare Innovation.
Dr. Kelly leads a pan-Canadian Clinical Trials Training Platform, called Increasing Capacity for Maternal and Paediatric Clinical Trials, known as IMPaCT. She is also the Scientific Director for the Canadian Collaborative for Childhood Cannabinoid Therapeutics, C4T.
As an expert in pediatric clinical trials and pharmacovigilance, Dr. Kelly has advisory roles with KidsCAN Trials, RareKids-CAN, and Conect4Children in Europe. Dr. Kelly is a former member of the Scientific Advisory Committee on Health Products Containing Cannabis at Health Canada, co-lead of the Canadian Medical Cannabis Trials Network and a current member of the Canadian Consortium for the Investigation of Cannabinoids Board of Directors.

Jonathan Kimmelman, PhD, is James McGill Professor of Biomedical Ethics at McGill University. His research group, STREAM (Studies in Translation, Ethics and Medicine), uses empirical and theoretical methods to understand the ethical, policy, and scientific dynamics of developing new drugs.
Kimmelman received the Maud Menten New Investigator Prize (2006), a CIHR New Investigator Award (2008), a Humboldt Bessel Award (2014), and was elected a Hastings Center Fellow (2018).
He has sat on various advisory bodies within the U.S. NHLBI and NIAID, served for four tours of duty on U.S. National Academies of Medicine committees, and chaired the International Society of Stem Cell Research Guidelines for Stem Cell Research and Clinical Translation revision task force 2015-16. His research has been covered in major media outlets, including NPR’s All Things Considered, STATNews, and Nature. Kimmelman is deputy editor at Clinical Trials, and associate editor at Med.
Knowledge Users (KUs) – ACT Clinical Trial Unit (CTU Leads)
ACT CTU scientific leads are KUs and early adopters for this project, as our research output will inform their practice as trialists in Canada. Other KUs are specialists in Maternal-Fetal Medicine and obstetric medicine.

Dr. Thierry Lacaze-Masmonteil is the Scientific Director of the Maternal Infant Child and Youth Research Network (MICYRN), a role he has held since May 2018. He earned his medical degree from the University Paris 5 – René Descartes in 1993, followed by a PhD in Biological Sciences from the University Paris 7 – Pierre et Marie Curie in 1995. He completed a Neonatology fellowship in 1997 and obtained a Master’s degree in Epidemiology in 2000. That same year, he was appointed Professor of Pediatrics at the University Paris 11.
In 2003, Dr. Lacaze-Masmonteil moved to Edmonton, Alberta, and in 2006 became the inaugural Director of the Women and Children’s Health Research Institute (WCHRI). He was later recruited as a Senior Scientist at the Children’s Hospital of Eastern Ontario (CHEO) Research Institute in 2010 and served as the Scientific Director of CHEO’s Clinical Research Unit from 2011 to 2015.
From 2016 to 2021, he served as Section Head of Neonatology at the Cumming School of Medicine, University of Calgary, and Regional Program Director of Neonatology at Alberta Health Services. Since May 2021, he has focused primarily on leading and expanding MICYRN, strengthening its role as an Academic Research Organization supporting pediatric and maternal health research.
Dr. Lacaze-Masmonteil is also Principal Investigator of the CIHR-funded CHEER initiative (Canadian Collaboration for Child Health: Efficiency and Excellence in the Ethics Review of Research). His research interests focus on pediatric clinical trials, with particular attention to drug development and innovative research methodologies.

Dr. Stephen Lapinsky is a Pulmonary and Critical Care physician at Mount Sinai Hospital in Toronto and a Professor of Medicine at the University of Toronto. He completed his training in Internal Medicine and Pulmonary/Critical Care Medicine in Johannesburg and has been a staff physician at Mount Sinai Hospital since 1994. From 2004 to 2020, he served as Director of the Intensive Care Unit.
Dr. Lapinsky’s clinical and research focus lies in the management of critical illness and pulmonary disease during pregnancy. He has authored over 60 research papers, reviews, editorials, and book chapters in this area. He is the Immediate Past President of the North American Society for Obstetric Medicine (NASOM) and currently serves as co-Editor-in-Chief of the journal Obstetric Medicine.

Dr. Scott Halperin is a Professor of Pediatrics and Microbiology & Immunology at Dalhousie University in Halifax, Nova Scotia. He serves as Director of the Canadian Center for Vaccinology (CCfV) and is the Nominated Principal Investigator of the Canadian Immunization Research Network (CIRN). He previously held the role of Co-Principal Investigator for the Immunization Monitoring Program–Active (IMPACT) and was a member of the Executive Committee of the Canadian Association for Immunization Research and Evaluation (CAIRE).
Dr. Halperin has played a pivotal role in building and leading collaborative vaccine research networks across Canada, generating research that directly informs public health policy and immunization practices. A leading expert in pertussis, his work spans the diagnosis, treatment, and prevention of pertussis and other vaccine-preventable diseases.
His research team has conducted more than 250 clinical trials, including over 20 phase 1 studies. These have covered early-phase vaccine trials for SARS-CoV-2, Ebola virus, group A Streptococcus, Bordetella pertussis, influenza, and respiratory syncytial virus (RSV), as well as later-phase studies for HPV, varicella-zoster virus, meningococcal B, and hepatitis B. Dr. Halperin is a trusted advisor to the vaccine industry and has served on numerous advisory committees for vaccine manufacturers.
Early-Career Researchers and Trainees
Our team includes the perspectives of trainees and successful maternal health early-career researchers.

Dr. Elisabeth McClymont is an Assistant Professor in the Departments of Obstetrics & Gynecology and Pediatrics at the University of British Columbia (UBC).
She earned her PhD in Reproductive and Developmental Sciences from UBC, followed by postdoctoral fellowships in Obstetrics & Gynecology and Pediatrics at both Université de Montréal and UBC. She also completed advanced postdoctoral training at the Danish Cancer Research Center in Denmark.
Dr. McClymont’s research focuses on reducing the burden of viral diseases in women and their infants. She has been the recipient of the Dr. Bernard Duval Award for Vaccination and the Young Investigator of the Year Award from the Infectious Diseases Society for Obstetrics and Gynecology (IDSOG). She currently serves as a Board Member of the International Papillomavirus Society.

Dr. Sonia Grandi, MSc, PhD, is a Research Scientist in the Child Evaluative Sciences Program at the Research Institute of the Hospital for Sick Children and an Assistant Professor in the Division of Epidemiology at the Dalla Lana School of Public Health, University of Toronto.
She earned her PhD in Epidemiology from McGill University and completed a postdoctoral fellowship in reproductive epidemiology at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the U.S. National Institutes of Health.
Dr. Grandi’s research focuses on improving maternal and child health across the life course. Her work has advanced understanding of how adverse pregnancy outcomes affect long-term maternal health, identified strategies for early identification and care of high-risk women, and strengthened the evidence base for medication use during pregnancy, particularly for women with cardiometabolic and immune-mediated conditions. She is also interested in the application of advanced methods for causal inference and using administrative health data to inform clinical care and decision-making.
Persons with Lived and Living Experiences (PWLLEs)
Patient partners to co-develop PREGTRIAL priorities, and will engage with other PWLLEs through the National Collaborating Centre for Methods and Tools (NCCMT) during the question development and refinement process, and via ad-hoc focus groups through our partnership with Pfizer Canada.
Collaborators










Oversight, Engagement and Synergy
Our team has expertise in patient-oriented research and consensus building. Together we will ensure engagement and synergy between PregTrial core team members, stakeholders/collaborators, and people with lives and living experience through our established governance structure.
PREGTRIAL Team Governance and Engagement Structure