Work Plan
Background and Rationale
The exclusion of pregnant and lactating individuals from clinical trials stems from concerns over fetal safety, a legacy of past medical tragedies like the thalidomide disaster. However, this exclusion perpetuates inequities, particularly among racialized and marginalized groups, who experience worse maternal and neonatal outcomes due to underrepresentation in research.
Without trial data, healthcare providers rely on limited evidence, often leading to suboptimal care. PREGTRIAL’s work addresses this gap by integrating ethical principles, reducing barriers to inclusion, and fostering a more inclusive research culture, ensuring better health outcomes for pregnant individuals and their infants.
Our Objective
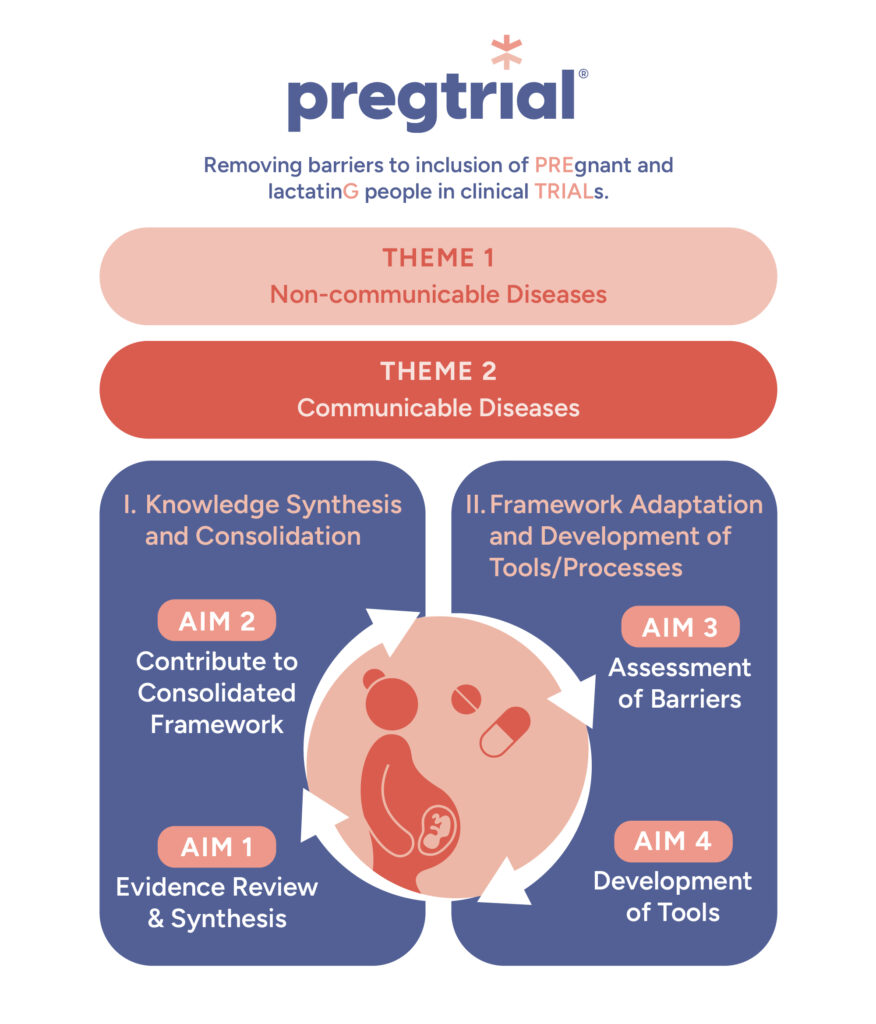
PREGTRIAL’s research plan is built on the Consolidated Framework for Implementation Research (CFIR) to create a comprehensive and practical foundation for including pregnant and lactating individuals in clinical trials.
Our overall objective is to generate an evidence-informed foundation upon which to implement ethical frameworks that support the safe inclusion of pregnant and lactating people in Canadian clinical trials. We will synthesize and consolidate knowledge, and develop concrete tools adapted to Canadian realities to achieve our overall objective.
Our Aims
Our Aims and Approach
We are reviewing evidence from available clinical trials over the last decade on inclusion or exclusion of pregnant and lactating people and of reproductive aged women. This will help us understand current practices and advocate for future change.
Support the development of Canada-specific guidance for the conduct of clinical trials including pregnant and lactating people.
Assess investigator and research ethics board-related barriers, facilitators, and needs to support the responsible inclusion of pregnant and lactating people in clinical trials.
Development of
Tools
We are co-developing tools such as an adapted informed consent form template and other toolkits using a multi-pronged approach to ensure that the ethics surrounding inclusion of pregnant and lactating people are adequately captured in contemporary research.
Knowledge Mobilization Framework
Our Knowledge Mobilization Framework focuses on coordinating different stakeholder groups, ensuring the impact of our research is far-reaching and inclusive.
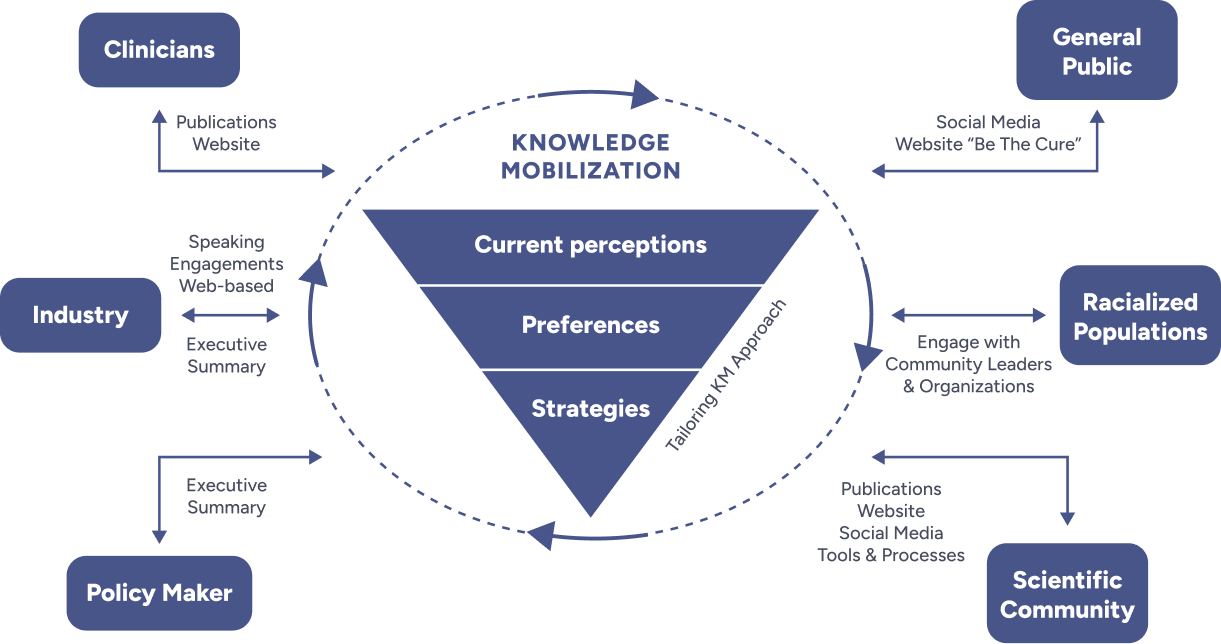
This framework ensures that ethical, scientific, and logistical challenges are addressed while fostering collaboration among researchers, stakeholders, and people with lived experience.